The genetic revolution is upon us. By making genetic tests more available and affordable, employers and insurers can capitalize on this movement and improve employee wellbeing and lower morbidity and mortality.
Professor Dame Sally C. Davies, the Chief Medical Officer of the Department of Health in the U.K. recently made a bold statement in her 2016 Annual Report entitled "Generation Genome", where she said, "Genomics is not tomorrow. It's here today.[i]"
In an accompanying video by the Telegraph she asks, "Please, can we now move from talking and experimenting around genomes and put it into practice for patients?"[ii] We sat down with Dr. Phil Smalley, Chief Medical Director of Wamberg Genomics Advisors, to ask how genomics can be added to employee benefits and incorporated into insurance products to further champion this noble cause to get more of the public informed about their genetics.
This will allow people to take a more proactive stance working with their doctors to prevent disease, avoid drug side effects and improve survival outcomes through personalized therapies for cancer. Also, this direction will rapidly accelerate research as more people enter much-needed clinical trials.
CWM: Are employers and insurance companies already offering genetic testing services?
Dr. Smalley: Yes. Employers have already started offering genetic testing services to employees in the U.S. and internationally. [iii],[iv] Companies have also made cancer genomic profiling services available to their employees in the U.S.
CWM: What are the types of genetic tests and how accurate are they?
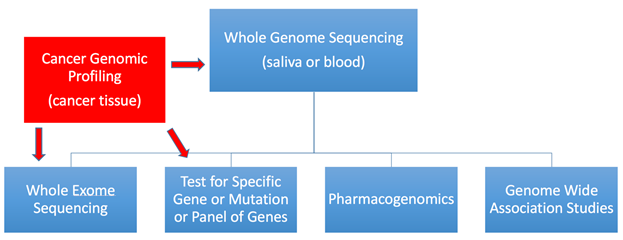
Dr. Smalley: There are a number of genetic tests available (see figure 1 for more information). Genetic testing done in certified laboratories is very accurate but not perfect; false positives and false negatives can still occur. What varies quite a bit is how predictive these tests are for developing a disease.
This predictability depends on the gene and the disease. In general, for the multifactorial multigene diseases like cardiovascular disease, the risk associated with any specific genetic variant or mutation is relatively low. Although there are some rare monogenetic diseases where having the mutation is 100 percent predictive that you will get the disease such as Huntington's Disease.
Lifestyle and environment also play important role modifying your genetic risk.[v] Most importantly, your genes are not fate. There are many preventative measures through increased screening, medications and/or surgeries that can dramatically decrease your risk of developing a disease even if you are carrying a genetic mutation.
Knowing your genetics might also help incentivize you to adopt a healthier lifestyle. Some studies show that after informing people of their genetic risks, the success rate of long-term lifestyle change is disappointing.[vi] But, when genetic testing has been incorporated into a multifaceted individualized corporate wellness program, we can enhance program engagement, promote weight loss and decrease annual health care costs. [vii]
Even though your genetics report states you don't carry a specific genetic mutation, you can still develop a disease. Also, even though your genetics/DNA stays fairly constant over your lifespan, the report of your genetics can change over time as researchers find new gene-disease risk associations. It is also important to remember that genetic tests must be interpreted as a whole, factoring in family history, ethnicity, and the person's own history and lifestyle.
It is always best to talk to your doctor and/or genetics counselor to fully understand the meaning of your test results for you as an individual.
CWM: How is genetic testing being used in clinical practice?
Dr. Smalley: Genetic tests are already in use in clinical medicine to diagnose monogenetic diseases such as cystic fibrosis, polycystic kidney disease, muscular dystrophy and much more. The U.S. Food & Drug Administration (FDA) recommends that genetic testing is performed before prescribing a handful of medicines to avoid drug side effects. [viii]
The PharmGKB professionally curated website reports on greater than 150 other medicines with actionable drug-gene interactions that are left up to the physician to decide whether to get pharmacogenomics testing done on their patients or not.[ix]One of the barriers to more widespread use of pharmacogenomics in clinical practice is that these tests are not typically reimbursed by health insurance payers.
Regarding predictive genetic tests, there are well-accepted clinical guidelines where some patients should get genetic testing performed, especially if there is a family history of a disease.[x] In the cancer space, a 2017 survey shows that 64 percent of 132 cancer specialists in the U.S. feel that genetic cancer genomic testing is useful now and many use genetic tests quite often, especially in cases of advanced cancer. [xi]
To date, 6 percent of the U.S. population has had some form of genetic testing performed, and 81 percent found the information useful. [xii] Expanding the accessibility and depth of genetic testing puts us slightly ahead of the clinical medicine curve. Many experts are calling for more research before widespread adoption of genetic testing in public health. [xiii], [xiv]
Getting genetic testing through one's employer or after insurance policy issue will take away some of the barriers of cost and time delay that currently impede the wider adoption of genomics in clinical medicine. Many surveys have shown that a significant proportion of the public and employees want access to their genetic information as they feel this information will help improve their wellbeing. For example, the 2017 Sanofi Canada Healthcare Survey reports that 67 percent of all employee members are interested in getting pharmacogenomic testing. [xv]
CWM: What is the value in offering employees Pharmacogenomic Testing?
Dr. Smalley: Many employees take prescription medications. [xvi] Unfortunately, many medications that doctors prescribe are ineffective. [xvii] Also, it has been shown that 3.5 percent of all hospitalizations in Europe are due to an adverse drug reaction.[xviii] In the U.S., serious adverse drug reactions are estimated to occur in about 2 million Americans a year and cause about 100,000 deaths annually, making it the fourth leading cause of death. [xix]
Pharmacogenomic testing allows doctors to prescribe the right drug, at the right dose to the right patient. More than 91 percent of people carry at least one actionable drug-gene mutation that if known would mean a doctor should use a different drug or altered dose to treat that person. [xx] Truly, one size does not fit all. Using genetic guided prescribing makes drug therapy more effective and helps avoid serious drug side effects.
CWM: What is the value in offering employees Cancer Genomic Profiling?
Dr. Smalley: Everyone has been touched by cancer in some way. We all have a 40 percent chance of developing cancer in our lifetime, and cancer causes more than 22 percent of all deaths. [xxi], [xxii] Genetic testing allows doctors to better prevent and treat cancer.
Cancer is a genetic disease and deserves a proper genetic diagnosis to allow the use of the most appropriate therapy and lead to new knowledge and better drugs through clinical trials.[xxiii]Many studies have shown that personalized cancer therapies based on tumor genetic biomarkers improve patient outcomes. [xxiv],[xxv], [xxvi]
In May 2017, the FDA took a novel direction by approving the use of a cancer immunotherapy drug based solely on the cancer's genetic mutation, regardless of where the cancer originated. [xxvii] There are also drugs today that are indicated for one form of cancer that could be applied to other forms of cancer once the genomic profile of a cancer is known.
Equally, other therapies such as immunotherapy can be targeted where they can be most effective. Even in end-stage cancer patients, some studies show that cancer genomic profiling guided targeted therapies can benefit patients. For example, Spetzler's 2015 study showed that genetic testing influenced treatment decision in 53 percent of patients and targeted therapies based on cancer genomic profiling extended life expectancy by 1.1 years. [xxviii]
Le Tourneau's 2015 SHIVA trial did not show a significant improvement in progression-free survival, but this small study has been questioned because of the treatments chosen and other study limitations. [xxix] I need to state that many of these studies are aimed at terminally ill, heavily pre-treated cancer patients. Application of genomic profiling earlier in the course of the disease should lead to a more favorable impact on cancer survival.
Getting cancer genetic profiling also allows patients to take part in various clinical trials such as large national cancer agency sponsored trials such as NCI-MATCH and ASCO-TAPUR trials and much more. [xxx] Cancer genetic profiling can also help a doctor avoid the use of expensive and toxic chemotherapy in some settings.
For example, in early stage breast cancer, genetic testing of clinically high-risk patients can lead to a 46 percent reduction in the number of patients needing additional post-surgery chemotherapy. [xxxi]

CWM: What is the value in offering employees Whole Genome Sequencing?
Dr. Smalley: Even though all humans are 99.5 percent the same genetically, we are all carrying various genetic mutations and alterations that put us at either an increased or decreased risk of developing various diseases. In the healthy population, 2.0 percent of adults of European ancestry and 1.1 percent of adults of African ancestry are walking around with incidental actionable genetic mutations that if known, could allow your doctor to take measures to prevent disease. [xxxii]
Some researchers have questioned the value of routine genetic testing of the public, but they still report that genetic testing uncovered some patients who had undiagnosed medical conditions. They also found that primary care providers are able to manage the results of genetic testing appropriately. xiv
CWM: What are the possible benefits of getting genetic testing done?
Dr. Smalley: The possible benefits are the following:
- Improve employee health resulting in less disability, better morale, less absenteeism, better productivity and increased company loyalty
- Prevention, early detection, and treatment of disease
- Extend quality and quantity of life
- Public taking an active role in their healthcare
- Encourage healthy behavior
- Peace of mind
- Could lead to new treatments and drug discovery
- Avoid drug side effects and more effective therapies
- Stop useless drug therapies and avoid toxic chemotherapies
- Family planning
- Lower health care cost
CWM: What are the potential concerns with getting genetic testing done?
Dr. Smalley: There are a few potential concerns, including:
- If tests are done outside of medical supervision, it is possible to misguide the public
- False positives and false negatives are possible
- False sense of security leading to patients not following doctor's suggestions
- Patients taking medication changes on their own without consulting their doctor
- Difficulty interpreting genetic variants of uncertain or unknown significance
- Might cause anxiety or fatalistic view
- Overzealous optimism for hope of cure
- Need for longitudinal follow-up to update the employee's report based on new discoveries in the future
- Most genetic variations are associated with relatively small relative risks and not very predictive
- Family strain
- Privacy and discrimination concerns
- Insurability concerns
- Insurance anti-selection but more so with whole-genome sequencing and not really a problem with pharmacogenomic testing and cancer genetic profiling
- Increase use of healthcare in follow-up
- Accessibility and cost of targeted cancer therapies
- Cost of testing
CWM: How will getting genetic testing done impact healthcare costs?
Dr. Smalley: The impact on healthcare costs depends on the circumstances and test. Pharmacogenomic testing can lower health care costs. For example, one study in patients with depression, genetic testing guided therapy can save an estimated US$1,035 per person per year in drug costs [xxxiii] Genetics guided therapy in the elderly polypharmacy patients can get patients off medications and can save an estimated US$621 per patient per year. [xxxiv]
Also, as serious adverse drug reactions are a common cause of emergency room visits, hospitalization and increased the length of stay in the hospital, using genetic guided medication prescribing that decreases drug side effects will lower overall health care costs considerably.
Whole genome sequencing might lead to a short-term 30 percent increase in healthcare spending in the first 6 months after testing as patients see their doctors to change therapy and get follow-up testing performed. xiv After this period, genetic testing will lead to more disease prevention therapies and improved lifestyle which result in less time off work with lower morbidity and mortality that saves costs to the employer and health insurer.
As cancer genomic profiling is fairly new, the net impact on healthcare costs is unclear currently. The cost of genetic testing leading to the use of expensive targeted therapies is mitigated by the cost savings attributed to being able to withhold additional chemotherapy, avoid expensive high-grade treatment-related toxicities that are more common in patients treated with conventional chemotherapy and lower the risk of cancer recurrence. [xxxv], [xxxvi]
CWM: What is the benefit using an intermediary like Wamberg Genomic Advisors (WGA) rather than just approaching the genetic testing service providers?
Dr. Smalley: Using an intermediary will ensure privacy for employees at the time of claim because no health or genetic information identifying the employee is given back to the employer. WGA also gives clients expert counsel to educate the employer about pros and cons of various types of genetic testing offerings and knowledge about good vendors that are appropriately vetted by our experts.
WGA also offers customized offerings with flexible funding and pricing options. We also offer dedicated implementation, account management, and client support. We assist with communication and enrollment process for the employees. Also as an intermediary that has access to multiple providers, we can ensure your employees get the appropriate testing as their doctor requests.
Also, pricing is reduced for the employer as we get bulk testing rates from providers. We also make it easy for an employer to start small and expand their genetic testing offering to their employees at a later date without having to negotiate deals with several separate testing service providers. [xxxvii]
Conclusion:
You can read more about the use of genetic testing in employee benefits in a 12 part article series WGA is publishing monthly; you can read the first one here.We invite you to answer this anonymous one question online survey and see what others think about genetic testing as an employee benefit. Also, post your comments and opinions in the comments section below as we start this open discussion.
Medical Disclaimer
All content in this article was created for informational purposes only. The content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician, genetic counselor or other qualified health provider with any questions you may have regarding a medical condition or testing. Never disregard professional medical advice or delay in seeking it because of something you have read in this article. Reliance on any information provided in this article is solely at your own risk.
Citations
[i] Prof. Dame Sally C. Davies, Annual Report of the Chief Medical Officer 2016, Generation Genome, London: Department of Health (2017), https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/631043/CMO_annual_report_generation_genome.pdf, Accessed September 12, 2017
[ii] Sarah Knapton, The Telegraph, Science, July 4, 2017, http://www.telegraph.co.uk/science/2017/07/03/chief-medical-officer-every-cancer-patient-should-have-dna-tested/, Accessed September 12, 2017
[iii] Andie Burjek, Genetic Testing Gets Toothy as a Workplace Benefit, Workforce; November 30, 2016, http://www.workforce.com/2016/11/30/genetic-testing-gets-toothy-test-workplace-benefit/, Accessed September 12, 2017
[iv] Gavin Teo, The second coming of consumer genomics with 3 predictions for 2018, MedCityNews, July 26, 2017, http://medcitynews.com/2017/07/second-coming-consumer-genomics-3-predictions-2018/, Accessed September 12, 2017
[v] Khera A et al. Genetic Risk, Adherence to a Healthy Lifestyle, and Coronary Disease. N Engl J Med. 2016 Dec 15;375(24):2349-2358
[vi] Hollands GJ et al, The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ. 2016 Mar 15;352:i1102
[vii] Steinberg G et al, Reducing Metabolic Syndrome Risk Using a Personalized Wellness Program. J Occup Environ Med. 2015 Dec;57(12):1269-74
[viii] U.S. Food and Drug Administration, http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm, Accessed September 12, 2017
[ix] PharmGKD Website; M. Whirl-Carrillo, E.M. McDonagh, J. M. Hebert, L. Gong, K. Sangkuhl, C.F. Thorn, R.B. Altman and T.E. Klein. “Pharmacogenomics Knowledge for Personalized Medicine” Clinical Pharmacology & Therapeutics (2012) 92(4): 414-417., https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3660037/pdf/nihms465364.pdf, https://www.pharmgkb.org/labels, Accessed September 12, 2017
[x] NCCN Guidelines Version 2.2017 Panel Members Genetic/Familial High-Risk Assessment: Breast and Ovarian, https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf, Accessed September 12, 2017
[xi] H. Jack West, MD and Gabriel Miller, Genomic Testing and Precision Medicine in Cancer Care, Medscape Internal Medicine; May 2, 2017, http://www.medscape.com/slideshow/genomics-and-oncology-report-6008655, Accessed September 12, 2017
[xii] Robert J. Blendon, Sc.D., Richard L. Menschel Professor of Health Policy and Political Analysis at Harvard T.H. Chan School of Public Health, Gideon Gil, Managing Editor, Enterprise and Partnerships of Stat and John M. Benson, Mary T. Gorski, and Justin M. Sayde at Harvard T.H. Chan School of Public Health; Stat January 2016, https://cdn1.sph.harvard.edu/wp-content/uploads/sites/94/2016/01/STAT-Harvard-Poll-Jan-2016-Genetic-Technology.pdf, Accessed September 12, 2017
[xiii] West HJ. No Solid Evidence, Only Hollow Argument for Universal Tumor Sequencing: Show Me the Data; JAMA Oncol. 2016 Jun 1;2(6):717-8
[xiv] Vassy JL et al, The Impact of Whole-Genome Sequencing on the Primary Care and Outcomes of Healthy Adult Patients: A Pilot Randomized Trial. Ann Intern Med. 2017 Jun 27;167:159-169
[xv] The Sanofi Canada Healthcare Survey 2017, Published by Transcontinental Media G.P., http://www.sanofi.ca/l/ca/en/layout.jsp?cnt=65B67ABD-BEF6-487B-8FC1-5D06FF8568ED, Accessed September 12, 2017
[xvi] National Center for Health Statistics. Health, United States, 2016: Chartbook on Long-term Trends in Health. Hyattsville, MD. 2017, https://www.cdc.gov/nchs/data/hus/hus16.pdf#079, Accessed September 13, 2017
[xvii] Schork NJ, Time for one-person trials; Nature. 2015 Apr 30;520(7549):609-11
[xviii] Bouvy JC. et al, Epidemiology of adverse drug reactions in Europe: a review of recent observational studies; Drug Saf. 2015 May;38(5):437-53
[xix] Karczewski KJ et al, Chapter 7: Pharmacogenomics. PLoS Comput Biol. 2012;8(12):e1002817
[xx] Carere DA et al, Prescription medication changes following direct-to-consumer personal genomic testing: findings from the Impact of Personal Genomics (PGen) Study. Genet Med. 2017 May;19(5):537-545
[xxi] National Cancer Institute Cancer Statistics- Updated March 22, 2017, https://www.cancer.gov/about-cancer/understanding/statistics, Accessed September 13, 2017
[xxii] Kenneth D. Kochanek et al, Deaths: Final Data for 2014; National Vital Statistics Reports June 30, 2016;65(4), https://www.cdc.gov/nchs/data/nvsr/nvsr65/nvsr65_04.pdf, Accessed September 13, 2017
[xxiii] Subbiah V and Kurzrock R, Universal Genomic Testing Needed to Win the War Against Cancer: Genomics IS the Diagnosis. JAMA Oncol. 2016 Jun 1;2(6):719-20
[xxiv] Schwaederle M et al, Association of Biomarker-Based Treatment Strategies With Response Rates and Progression-Free Survival in Refractory Malignant Neoplasms: A Meta-analysis. JAMA Oncol. 2016 Nov 1;2(11):1452-1459
[xxv] Schwaederle M et al, Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials. J Clin Oncol. 2015 Nov 10;33(32):3817-25
[xxvi] Massard C et al, High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017 Jun;7(6):586-595
[xxvii] U.S. Food and Drug Administration, https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm560167.htm, Accessed September 15, 2017
[xxviii] Spetzler D et al, Multi-platform molecular profiling of 1,180 patients increases median overall survival and influences treatment decision in 53% of cases. European Journal of Cancer, September 2015; Volume 51, Supplement 3, Page S44
[xxix] Le Tourneau C et al, Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015 Oct;16(13):1324-34
[xxx] ClinicalTrials.gov, A service of the U.S. National Institutes of Health; NCI-MATCH: Targeted Therapy Directed by Genetic Testing in Treating Patients with Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma; NCT02465060; ASCO-TAPUR: Testing the Use of Food and Drug Administration (FDA) Approved Drugs That Target a Specific Abnormality in a Tumor Gene in People with Advanced Stage Cancer; NCT02693535; https://clinicaltrials.gov, Accessed September 13, 2017
[xxxi] Cardoso F et al, 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016 Aug 25;375(8):717-29
[xxxii] Amendola LM et al, Actionable exomic incidental findings in 6503 participants: challenges of variant classification. Genome Res. 2015 Mar;25(3):305-15
[xxxiii] Winner JG et al, Combinatorial pharmacogenomic guidance for psychiatric medications reduces overall pharmacy costs in a 1 year prospective evaluation. Curr Med Res Opin. 2015;31(9):1633-43
[xxxiv] Saldivar JS et al, Initial assessment of the benefits of implementing pharmacogenetics into the medical management of patients in a long-term care facility. Pharmgenomics Pers Med. 2016 Jan 19;9:1-6
[xxxv] Haslem DS et al, A Retrospective Analysis of Precision Medicine Outcomes in Patients With Advanced Cancer Reveals Improved Progression-Free Survival Without Increased Health Care Costs.; J Oncol Pract, February 2017; Volume 13 (Issue 2): pp e108-e199
[xxxvi] Reck M et al, Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016 Nov 10;375(19):1823-1833
[xxxvii] Wamberg Genomic Advisors (WGA), www.wamberggenomic.com, Accessed September 13, 2017








